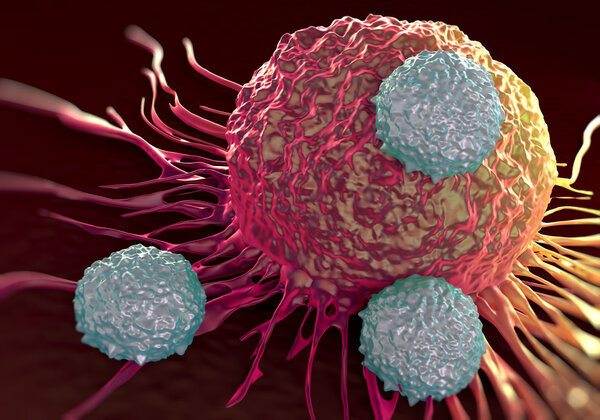
Once hailed as a "miracle drug" for cancer by the medical community, CAR-T therapy has recently come under investigation by the FDA due to safety concerns.
As the most authoritative drug regulatory agency in the world, the U.S. Food and Drug Administration (FDA) issued a notice on its official website announcing an investigation into the risk of patients developing T-cell malignancies (including chimeric antigen receptor (CAR) T-cell-positive lymphoma) after receiving autologous CAR-T cell immunotherapy targeting BCMA or CD19.